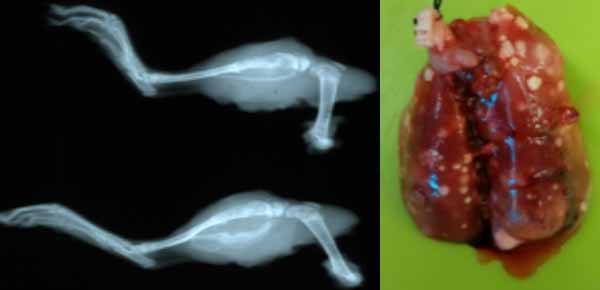
Research in oncology in our department is led by veterinary scientist/pathologist Dr. Kathy Gabrielson. She works with multiple investigators at Johns Hopkins University in assessing efficacy of experimental drugs on cancer in animal models. She is a PhD trained toxicologist and also assesses off target toxicity for other laboratories. In her own laboratory, she developed a cardioprotective strategy to block cancer therapeutic doxorubicin cardiac toxicity in a mouse model of breast cancer, and found that this strategy also increased cancer cell death. Recently, she developed a novel model to study the long latency effects of doxorubicin cardiomyopathy in a rat model of osteosarcoma. This is an immunocompetent, outbred Sprague-Dawley, syngeneic rat model with implanted UMR106 osteosarcoma cell line, with orthotopic tibial implants, who develop reproducible primary and metastatic pulmonary tumors. Using limb amputation, the Gabrielson lab is able to control the incidence of pulmonary metastasis in this animal model of pediatric osteosarcoma.
Dr. Gabrielson also routinely uses non-invasive in vivo imaging in these animal models and correlates and validates acquired images using gross and histopathology. With this background and experience, she collaborates with investigators across multiple departments at the School of Medicine at Johns Hopkins University to validate ultrasound, nuclear, optical and MRI in vivo images with gross and histopathology. She is the director of the JHU Miller Research Building Imaging Facility, Ultrasound lab and assists PIs with ultrasound for cancer, cardiac and developmental biology studies.
Dr. Gabrielson has a long history of cardiovascular research in her collaborations and in her own research program. In 1998, FDA approved the first therapeutic agent that molecularly targets a protein called HER2 or ErbB2. This therapeutic agent is trastuzuamb (Herceptin or anti-erbB2 or anti-HER2) and it specifically targets and inhibits ErbB2 as it is important in breast cancer pathogenesis. This therapeutic antibody has dramatically increased the long term survival of patients with HER2 overexpression in breast cancer but unfortunately it does cause heart toxicity especially when doxorubicin is given concurrently. Her lab then further studied the signal transduction pathways of this toxicity in the heart and found that the molecular target of trastuzumab (ErbB2) increases in the heart after doxorubicin therapy in the animal model thus shedding light on mechanisms of the toxicity. Since the role of ErbB2 in the heart is not understood, her lab developed a cardiac specific ErbB2 transgenic mouse model to further study ErbB2 and found that ErbB2 is involved in reducing oxidative stress in the heart.
Dr. Gabrielson is also interested in stress and its role in cardiovascular disease and cancer. Recently the Gabrielson lab discovered that ErbB2 is in a reciprocal regulation relationship with the beta-adrenergic receptors and increased in the heart during acute β-adrenergic stress and transactivated by chronic β-adrenergic stimulation in vivo in the heart. Recently, the Gabrielson lab has extended their interest in stress and while working with Juntendo University in Japan have created a model of prenatal stress in mice to study acceleration of atherosclerosis (Apo e – /- mice) and lung cancer (AJ mice) in mouse models.
Dr. Gabrielson’s lab developed a method of electrocardiographic (ECG) characterization in hearts of awake mice that can be used to electrocardiographically characterize mice cardiac hypertrophy. She has assisted laboratories at Johns Hopkins University in performing and interpreting EKGs in other animal models.
